Explain the Relationship Between Electronegativity Difference and Polarity
Students will plan to change one variable collect data and chart the data graphically. This focus will help the students understand bond polarity.

Difference Between Electronegativity And Polarity Compare The Difference Between Similar Terms
The concept of electronegativity was first proposed by Pauli in 1932 as an explanation of the fact.

. The electronegativity of an atom is affected by both its atomic number and the distance that its valence electrons reside from the charged nuclei. The experiment consists of a student-created scaled snow sled model going down a teacher-created ramp. Students will determine the difference between balanced and unbalanced forces through an experiment.
Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons toward itself. Students will change a variable such as. Enter the email address you signed up with and well email you a reset link.
Number of students riding the snow sled. The relationship between the difference in electronegativity and bond.
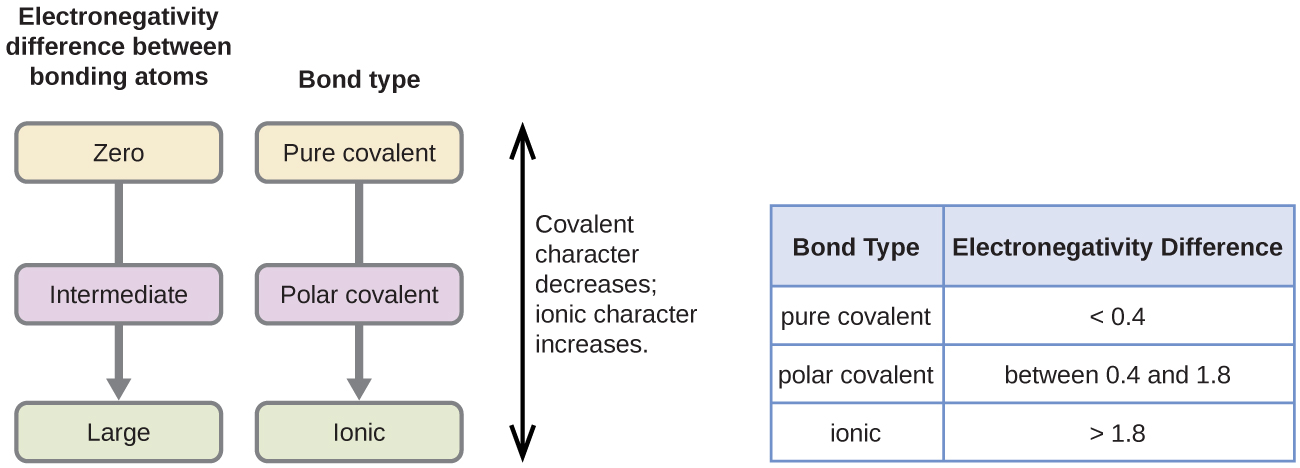
4 8 Polar Covalent Bonds And Electronegativity Chemistry Libretexts

Dublin Schools Lesson Electronegativity
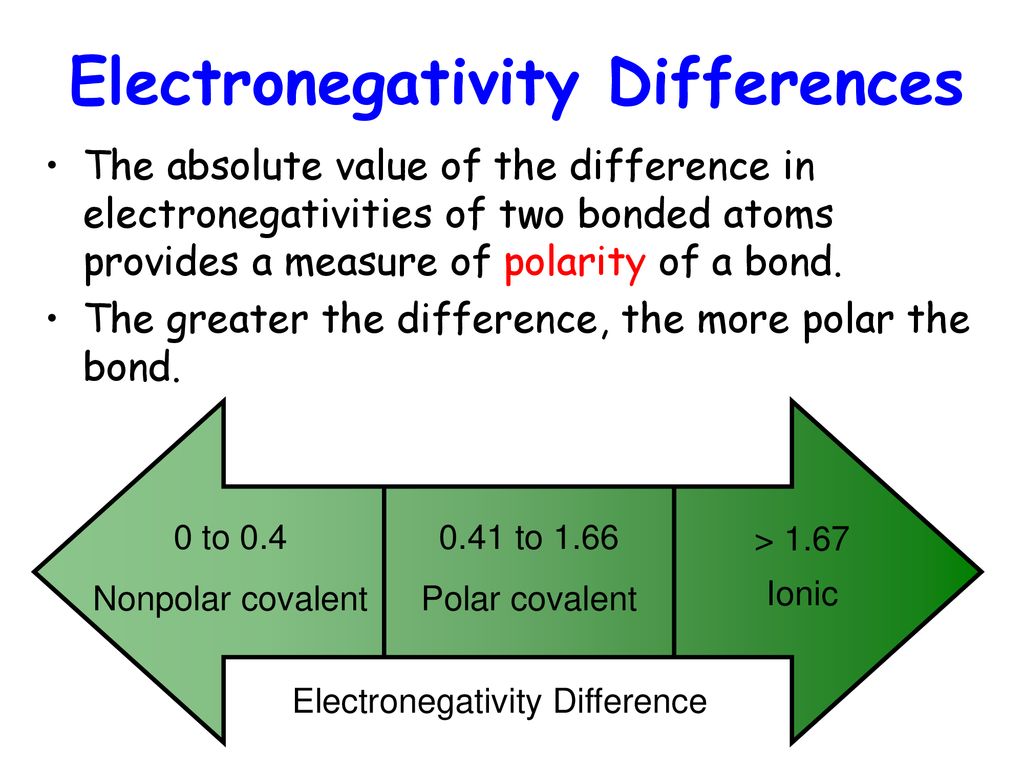

Comments
Post a Comment